Our laboratory primary research goals are directed toward understanding the complex interactions of infectious microorganisms with the immune system, as the balance in this interplay impacts whether host damage occurs.
We are interested in understanding three basic questions:
- Which mechanisms are used by microbes to invade, survive, and cause disease to the host?
- How does the host defend itself against infectious organisms?
- What is the role that the environment play in the evolution of microbial virulence?
We approach our research in an interdisciplinary manner, students and post-doctoral fellows that join the lab will be trained on basic microbiology, microscopy, immunological and tissue culture techniques, molecular biology, neuroscience, omics, therapeutic development research, and animal models of infection.
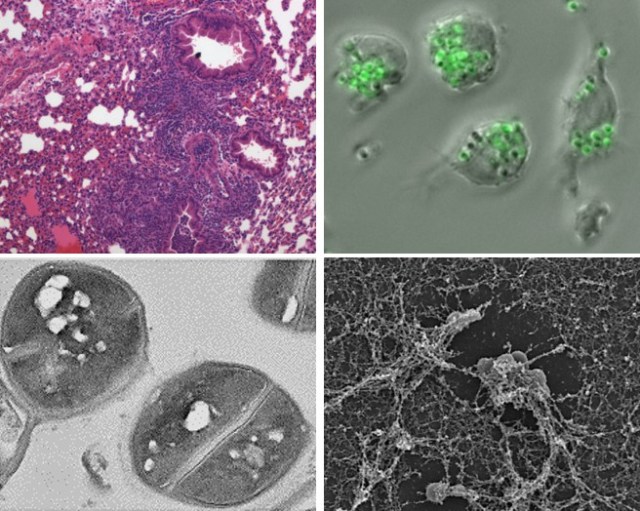
Cryptococcus neoformans is a global and neurotropic fungal pathogen.
The encapsulated fungus Cryptococcus neoformans is the most common cause of fungal meningitis, with the highest rate of disease in patients with AIDS. This microbe has developed its virulence factors by interacting with other organisms in the environment. It enters into the human body via the respiratory tract and the infection is controlled in the lungs of people with normal immunity. When a person’s immune system is defective, C. neoformans moves into the bloodstream and the fungus disseminates with a particular propensity to infect the brain. We are interested in elucidating the mechanisms of central nervous system (CNS) invasion by C. neoformans and the interactions of the fungus with cells of the CNS including microglia, astrocytes, and neurons.
Current projects include:
Unraveling C. neoformans mechanisms of brain invasion and colonization. C.neoformans’ capsule contributes directly to its pathogenesis, and we recently associated its main component, GXM, with C. neoformans’ ability to invade the brain via cerebral capillaries that comprise the blood-brain barrier (BBB). We have demonstrated that inhibiting GXM-mediated signaling in brain endothelial cells has similar positive effects as standard antifungal treatment in vivo, suggesting that maintaining BBB integrity is critical for preventing cryptococcal meningitis. The neurovascular unit (NVU) regulates and maintains BBB integrity through a continuous and dynamic interaction among its cellular components, namely endothelial cells, pericytes, astrocytes and microglia. Dysregulation of the NVU plays a critical role in the progression of neurocryptococcosis, which is characterized by major BBB breakdown. Therefore, our goal is to elucidate the cellular, molecular, and signaling mechanisms by which C. neoformans GXM dysregulates the BBB and invades the CNS. Since cryptococcal meningoencephalitis (CME) is often associated with AIDS-associated immunosuppression, we are comparing wild-type and CD4 T cell-deficient mice, which mimic the destruction of CD4+ T cells in HIV infection. We are testing the central hypotheses that C. neoformans directly disrupts the NVU function resulting in vascular damage and subsequent fungal invasion and CNS colonization and that C. neoformans GXM plays an essential role by interfering with NVU cell type specific processes. To test these hypotheses, we are investigating the following specific aims: (1) To identify cell-type specific genes that are differentially regulated in response to C. neoformans infection in cells from the NVU in immunocompetent vs. immunosuppressed mice; (2) To determine the dynamics of C. neoformans CNS invasion in conditions of immunosuppression in vivo; and (3) To determine how C. neoformans infection compromises the brain vasculature by affecting the NVU in CD4+ T cell depleted mice. Understanding the mechanisms behind cerebral cryptococcosis is significant to the development of therapies for its treatment.
Mechanisms underlying C. neoformans-induced meningoencephalitis and neurotoxicity. Post-mortem pathological studies in human CME brain tissue samples have demonstrated that there is an intimate relationship between neurons and cryptococcomas. However, the role of GXM on neuronal and cognitive dysfunction observed in CME patients is not well understood. Our preliminary data show that C. neoformans GXM binds to the surface of neurons and causes several cellular changes including morphological alterations, increases in intracellular calcium activity, and altered synaptic transmission. GXM release also leads to an increase in reactive astrocytes and hinders microglial migration, both findings that directly link immune response factors to C. neoformans neuropathology. Hence, our goal is to elucidate the mechanisms by which C. neoformans GXM interfere with neuronal physiology in the basal ganglia, one of the main brain regions associated with cognitive and motor function and significantly colonized by C. neoformans, especially in the setting of CD4 T cell deficiency. Our central hypothesis is that C. neoformans GXM accumulation results in neuroimmune dysregulation and neurotoxicity, contributing to CME pathogenesis and cognitive impairment. To address our hypothesis, we are investigating the following aims: (1) To determine how C. neoformans GXM affects basal ganglia function in CD4 T cell-deficient mice; (2) To test the hypothesis that exposure to C. neoformans GXM leads to basal ganglia principal neurons death in CD4 T cell-deficient mice; and (3) To investigate how C. neoformans exposure leads to altered synaptic transmission in the basal ganglia and lead to neurobehavioral deficits. Identifying novel mechanisms by which C. neoformans alters neuronal function and behavior in immunocompromised individuals will provide new insights into this neuropathology. In addition, results from our project will potentially facilitate future development of new therapeutics and preventive measures for combating CNS cryptococcosis (a disease that kills ~20% of people with AIDS worldwide) and effective management of its potential neuro-sequelae in survivors.
Role of astrocytes in C. neoformans meningoencephalitis during immunosuppression. Astrocytic activation occurring in response to various CNS insults plays a fundamental role in brain homeostasis and neurodegeneration. Reactive astrocytes are associated with destructive brain lesions filled with cryptococci and highly accumulated GXM in immunocompromised patients with CME. However, the role that astrocytes may play in the development of cerebral cryptococcosis is poorly understood. From a functional diversity standpoint, reactive astrocytes can be grouped in two phenotypes “A1” and “A2” cells. A1 reactive astrocytes (A1s) are associated with neuroinflammation whereas A2 reactive astrocytes (A2s) are presented in cases of inadequate blood supply or ischemia. Since CME is characterized by minimal inflammation, it is likely that A2s are predominantly activated during the disease. However, the molecular and cellular mechanisms underlying the emergence of astrocytic subpopulations during immunosuppression and CME are unknown. Aquaporin 4 (AQP4) is a water channel protein present in astrocytes with polarized distribution in endfeet membranes facing the perivascular complex. The strategic location at the blood-brain interface makes AQP4 an important factor in the regulation of water movement into and out of the brain. Our data shows drastic changes in AQP4 expression in the presence of GXM. Thus, we are investigating how C. neoformans infection and GXM accumulation causes astrocytic morphological changes, activation, and affect astroglia AQP4 expression, a critical water channel protein that contributes to brain edema leading to intracranial pressure (ICP). These two pathological complications are related to high CME mortality in individuals with AIDS. Our central hypothesis is that C. neoformans infection and GXM release induce A2s astrogliosis and disrupt AQP4, promoting C. neoformans brain colonization, and the development of CME, edema, and ICP. We propose the following aims: (1) To determine the specific astrocytic type that is activated by C. neoformans brain infection during immunosuppression; (2) Establish the role of C. neoformans GXM production in inducing astrogliosis and microglia dysregulation in CD4-depleted mice; and (3) Determine the role of AQP4 in edema development following C. neoformans brain invasion during immunosuppression. Identifying the mechanisms by which C. neoformans GXM alters astrocyte function will provide novel insights into the neurotropism of this deadly infection and may offer new therapeutic opportunities and preventive measures for combating CME, a disease that kills ~112,000 people per year worldwide.